- English
- French
- German
- Portuguese
- Spanish
- Russian
- Japanese
- Korean
- Arabic
- Greek
- German
- Turkish
- Italian
- Danish
- Romanian
- Indonesian
- Czech
- Afrikaans
- Swedish
- Polish
- Basque
- Catalan
- Esperanto
- Hindi
- Lao
- Albanian
- Amharic
- Armenian
- Azerbaijani
- Belarusian
- Bengali
- Bosnian
- Bulgarian
- Cebuano
- Chichewa
- Corsican
- Croatian
- Dutch
- Estonian
- Filipino
- Finnish
- Frisian
- Galician
- Georgian
- Gujarati
- Haitian
- Hausa
- Hawaiian
- Hebrew
- Hmong
- Hungarian
- Icelandic
- Igbo
- Javanese
- Kannada
- Kazakh
- Khmer
- Kurdish
- Kyrgyz
- Latin
- Latvian
- Lithuanian
- Luxembou..
- Macedonian
- Malagasy
- Malay
- Malayalam
- Maltese
- Maori
- Marathi
- Mongolian
- Burmese
- Nepali
- Norwegian
- Pashto
- Persian
- Punjabi
- Serbian
- Sesotho
- Sinhala
- Slovak
- Slovenian
- Somali
- Samoan
- Scots Gaelic
- Shona
- Sindhi
- Sundanese
- Swahili
- Tajik
- Tamil
- Telugu
- Thai
- Ukrainian
- Urdu
- Uzbek
- Vietnamese
- Welsh
- Xhosa
- Yiddish
- Yoruba
- Zulu
How can flexible sensors achieve long-term stability?
In implantable medical devices such as pacemakers and insulin pumps, the flexible temperature sensor of implantable devices has become the core component of postoperative monitoring due to its "biocompatibility+long-term stability" characteristics
H2: Special requirements for implantable device temperature measurement
H3: Biocompatibility (requires ISO 10993 cytotoxicity testing)
H3: Long term stability (no sexual decline for more than 5 years)
H3: Ultra thin and flexible (avoiding compression of surrounding tissues)
H2: Advantages of Implantable Scenarios for Flexible Sensors

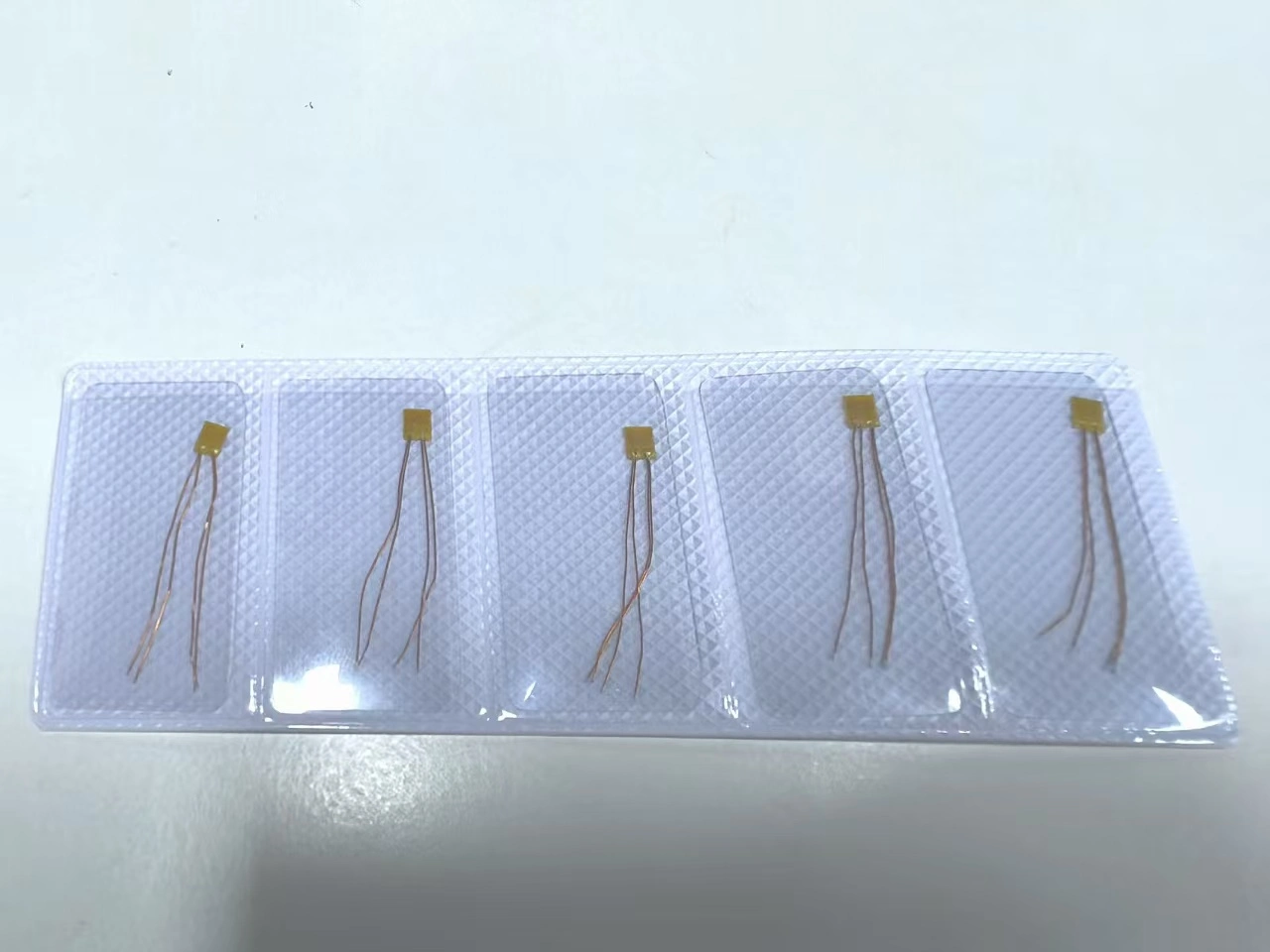
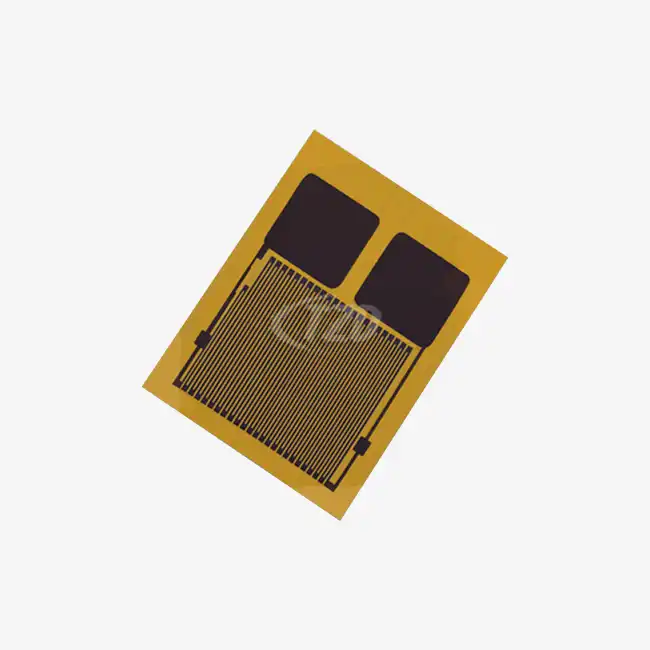
Our implantable device flexible temperature sensor adopts a 0.05mm medical grade PI substrate+latex free coating, certified by ISO 10993, with a cytotoxicity level of 0 (the highest safety level).
H3: Long term stability: Accuracy still ± 0.6 ℃ after accelerated aging test (85 ℃/85% RH, 1000 hours)
A certain insulin pump manufacturer's implant test showed that the sensor drift over 5 years was less than 0.2 ℃, which is a reliable proof of the flexible temperature sensor for implantable devices.
H3: Anti tissue adhesion: Smooth surface treatment (Ra ≤ 0.1 μ m) to reduce fiber wrapping
H2: Practical Case: Lepu Medical Cardiac Pacemaker Project
Scenario: Lepu's new generation of leadless pacemaker (implantation site: right ventricle)
Solution: Customized 3 × 2mm flexible sensor (integrated into electrode wire)
Effect: The accuracy of temperature monitoring at 3 months after surgery was 99.2%, with no tissue inflammatory response
H2: Certification and Quality Assurance: "Trust Endorsement" for Implantable Sensors
Core certifications: ISO 10993 (biocompatibility), NMPA filing (Class II medical devices), FDA 510 (k) (optional)
Warranty policy: 5-year free replacement (industry average of 3 years), lifetime technical support
References (3):
Biocompatibility Evaluation Standards for Implantable Medical Devices (GB/T 168862023)
Clinical Report of Lepu Medical Pacemaker Project (2024)
SGS Biocompatibility Test Report (Report Number: SZ-SGS-202404)
Conclusion+CTA (bold keywords): For implantable device manufacturers, implantable device flexible temperature sensors are the key to improving product safety and competitiveness. Apply for free samples now and experience 5 years of stable protection!
Author Information: Mr. Wang | Director of Implantable Sensor Technology (8 years of experience in medical implant device research and development, holding 3 invention patents)
FAQ (3 questions):
Q1: Can the sensor withstand corrosion from body fluids?
A: The substrate material is medical grade polyimide, which has passed the body fluid corrosion resistance test (pH 7.4, 37 ℃) for 180 days.
Q2: Do I need to replace it after implantation?
A: Design lifespan of 10 years, free replacement within 5 years without human damage.
Q3: Does it support wireless data transmission?
A: Can integrate micro Bluetooth modules (size increase ≤ 2mm), compatible with remote monitoring systems.

Xi'an Tongzida Technology Co., Ltd. is a leading manufacturer and supplier of advanced thermistor technology, dedicated to providing high-performance temperature sensors for demanding industrial applications. We have comprehensive expertise in microsensor manufacturing technology, special packaging technology, and multi-sensor integration methods to ensure excellent performance and reliability of thermistors. As a trusted supplier of precision temperature measurement solutions, we offer a complete thermistor system, including core sensitive chips, testing systems, and analysis software. Please contact our technical experts sales11@xatzd.com Discuss your specific thermistor requirements and learn how our advanced resistor temperature optimization and manufacturing capabilities can improve the performance and reliability of your temperature measurement system

Learn about our latest products and discounts through SMS or email